Nitric Oxide
Nitric oxide (NO) is produced by many cells in the body; however, its production by vascular endothelium is very important in regulating blood flow. Because of its importance in vascular function, abnormal production of NO, as occurs in different disease states or following vascular injury, can adversely affect blood flow and other vascular functions.
NO Biosynthesis
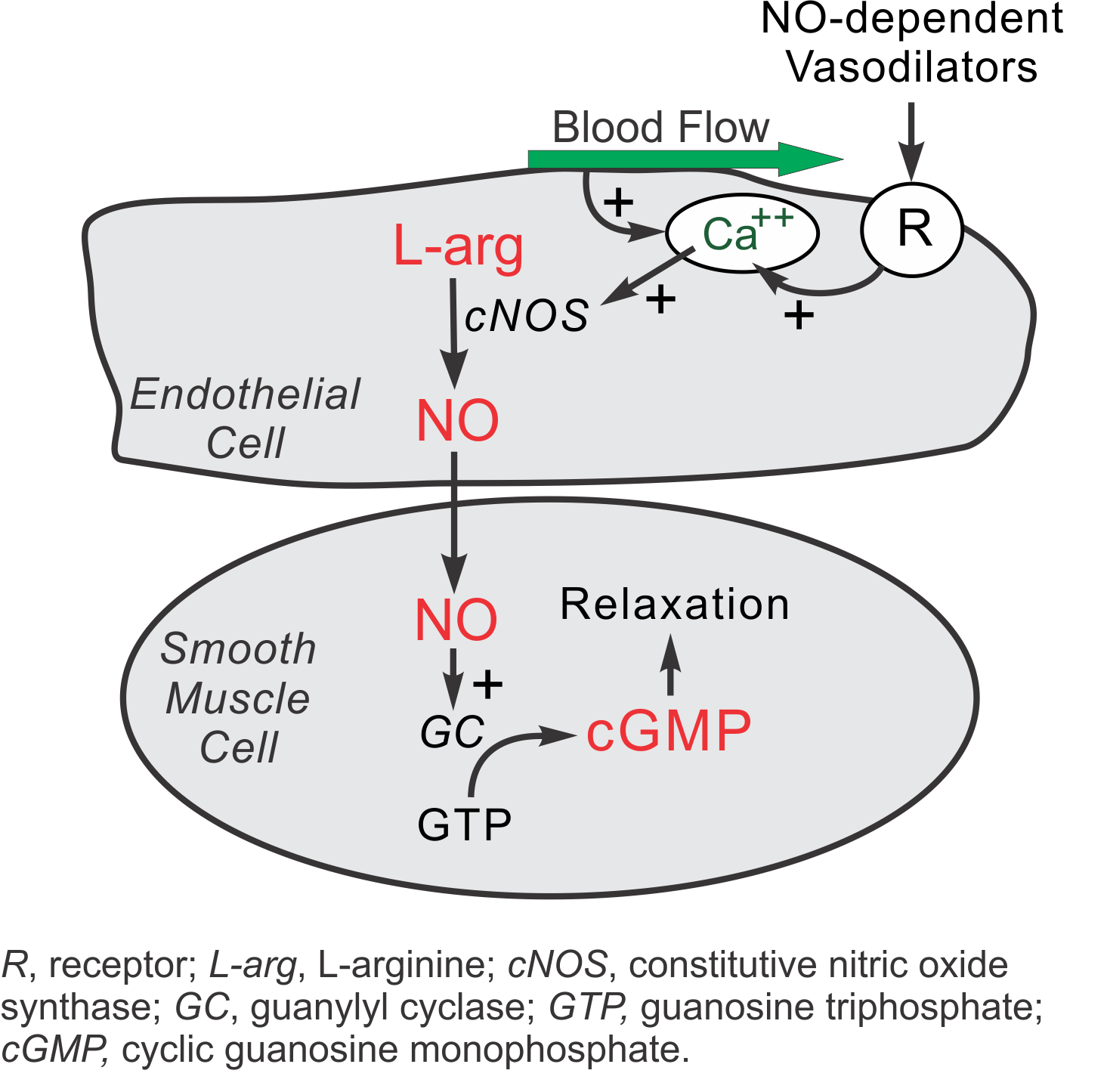 NO is produced from the amino acid L-arginine by the enzymatic action of nitric oxide synthase (NOS). There are two endothelial forms of NOS: constitutive NOS (cNOS, type III NOS) and inducible NOS (iNOS, type II NOS). Co-factors for NOS include oxygen, NADPH, tetrahydrobiopterin and flavin adenine nucleotides. Besides endothelial NOS, there is a NOS of neural origin (nNOS, type I) that serves as a transmitter in the brain and in different nerves of the peripheral nervous system, such as non-adrenergic, non-cholinergic (NANC) autonomic nerves that innervate penile erectile tissues and other specialized tissues in the body to produce vasodilation.
NO is produced from the amino acid L-arginine by the enzymatic action of nitric oxide synthase (NOS). There are two endothelial forms of NOS: constitutive NOS (cNOS, type III NOS) and inducible NOS (iNOS, type II NOS). Co-factors for NOS include oxygen, NADPH, tetrahydrobiopterin and flavin adenine nucleotides. Besides endothelial NOS, there is a NOS of neural origin (nNOS, type I) that serves as a transmitter in the brain and in different nerves of the peripheral nervous system, such as non-adrenergic, non-cholinergic (NANC) autonomic nerves that innervate penile erectile tissues and other specialized tissues in the body to produce vasodilation.
Under normal, basal conditions in blood vessels, NO is continually being produced by cNOS. The activity of cNOS is calcium- and calmodulin-dependent. There are two basic pathways for the stimulation of cNOS, both of which involve the release of calcium ions from subsarcolemmal storage sites. First, shearing forces acting on the vascular endothelium generated by blood flow causes a release of calcium and subsequent cNOS activation. Therefore, increases in blood flow stimulate NO formation (flow-dependent NO formation). Second, endothelial receptors for a variety of ligands stimulate calcium release and subsequent NO production (receptor-stimulated NO formation). Included are receptors for acetylcholine (M3 muscarinic), bradykinin, substance-P, adenosine, and many other vasoactive substances. In the late 1970s, Dr. Robert Furchgott observed that acetylcholine released a substance that produced vascular relaxation, but only when the endothelium was intact. This observation opened this field of research and eventually led to him receiving a Nobel prize. Initially, Furchgott called this substance endothelium-derived relaxing factor (EDRF), but by the mid-1980s he and others identified this substance as being NO.
A second isoform of endothelial NOS is iNOS. It differs, in part, from cNOS in that its activation is calcium independent. Under normal, basal conditions, the activity of iNOS is very low. The activity of iNOS is stimulated during inflammation by bacterial endotoxins (e.g., lipopolysaccharide) and cytokines such as tumor necrosis factor (TNF) and interleukins. During inflammation, the amount of NO produced by iNOS may be a 1,000-fold greater than that produced by cNOS.
Intracellular Mechanisms
When NO forms, it has a half-life of only a few seconds because superoxide anion has a high affinity for NO (both molecules have an unpaired electron, making them highly reactive). Therefore, superoxide anion reduces NO bioavailability. NO also avidly binds to the heme moiety of hemoglobin (in red blood cells) and the heme moiety of the enzyme guanylyl cyclase, which is found in vascular smooth muscle cells and most other cells of the body. Therefore, when NO is formed by vascular endothelium, it rapidly diffuses into the blood where it binds to hemoglobin and is subsequently broken down. It also diffuses into the vascular smooth muscle cells next to the endothelium where it binds to and activates guanylyl cyclase. This enzyme catalyzes the dephosphorylation of GTP to cGMP, which serves as a second messenger for many important cellular functions, particularly for signalling smooth muscle relaxation.
Cyclic GMP induces smooth muscle relaxation by multiple mechanisms, including
- Increased intracellular cGMP, which inhibits calcium entry into the cell, and decreases intracellular calcium concentrations (click here for details)
- Activating K+ channels, which leads to hyperpolarization and relaxation
- Stimulating a cGMP-dependent protein kinase that activates myosin light chain phosphatase, the enzyme that dephosphorylates myosin light chains, which leads to smooth muscle relaxation.
Because of the central role of cGMP in NO-mediated vasodilation, drugs (e.g., Viagra®) that inhibit the breakdown of cGMP (cGMP-dependent phosphodiesterase inhibitors) are used to enhance NO-mediated vasodilation, particularly in penile erectile tissue in the treatment of erectile dysfunction. Increased cGMP also has an important anti-platelet, anti-aggregatory effect.
Vascular Effects of NO
Vascular actions of NO include:
- Direct vasodilation (flow dependent and receptor mediated)
- Indirect vasodilation by inhibiting vasoconstrictor influences (e.g., inhibits angiotensin II and sympathetic vasoconstriction)
- Anti-thrombotic effect - inhibits platelet adhesion to the vascular endothelium
-
Anti-inflammatory effect - inhibits leukocyte adhesion to vascular endothelium; scavenges superoxide anion
- Anti-proliferative effect - inhibits smooth muscle hyperplasia
Because of the above actions of NO, when its production is impaired or its bioavailability is reduced, the following can result:
- Vasoconstriction (e.g., coronary vasospasm, elevated systemic vascular resistance, hypertension)
- Thrombosis because of platelet aggregation and adhesion to vascular endothelium
- Inflammation because of upregulation of leukocyte and endothelial adhesion molecules
- Vascular hypertrophy and stenosis
Diseases or Conditions Associated with Abnormal NO Production and Bioavailability
- Hypertension
- Obesity
- Dyslipidemias (particularly hypercholesterolemia and hypertriglyceridemia)
- Diabetes (both type I and II)
- Heart failure
- Atherosclerosis
- Aging
- Cigarette smoking
Revised 01/05/2018
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)