Reactive Oxygen Species
Reactive oxygen species (ROS) are highly reactive chemical forms of oxygen, such as superoxide anion and hydroxyl radical. There are several enzymes (oxidases) in the body that can form superoxide anion (⋅O2-) from molecular oxygen. As shown in the diagram below, NAD(P)H oxidase, cyclooxygenase (COX), xanthine oxidase (XO) and nitric oxide synthase (NOS) can form superoxide anion from molecular oxygen. Increased oxidative metabolism, the absence of certain oxidase cofactors (e.g., tetrahydrobiopterin required by NOS), and inflammatory and disease conditions can lead to increased superoxide production.
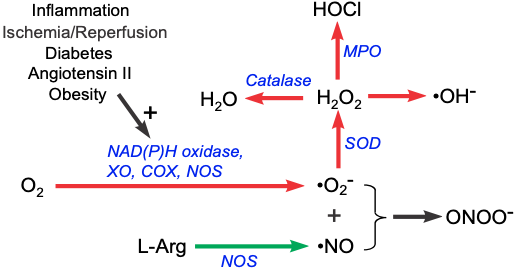 Superoxide anion is a highly reactive species that can damage cells. However, the body has scavenging and antioxidant mechanisms that can rapidly remove the superoxide anion. Superoxide dismutase (SOD) forms hydrogen peroxide (H2O2) from the superoxide anion. Catalase then converts hydrogen peroxide into water. Some hydrogen peroxide is acted upon by myeloperoxidase (MPO), an enzyme associated with polymorphonuclear leukocytes such as neutrophils, to form hypochlorous acid (HOCl), which can further form hydroxyl radicals. Hydrogen peroxide can also form hydroxyl-like compounds, many of which are highly reactive chemical entities.
Superoxide anion is a highly reactive species that can damage cells. However, the body has scavenging and antioxidant mechanisms that can rapidly remove the superoxide anion. Superoxide dismutase (SOD) forms hydrogen peroxide (H2O2) from the superoxide anion. Catalase then converts hydrogen peroxide into water. Some hydrogen peroxide is acted upon by myeloperoxidase (MPO), an enzyme associated with polymorphonuclear leukocytes such as neutrophils, to form hypochlorous acid (HOCl), which can further form hydroxyl radicals. Hydrogen peroxide can also form hydroxyl-like compounds, many of which are highly reactive chemical entities.
While superoxide and its products can have vasoactive activities besides their tissue damaging effects, superoxide anion has another property that makes it significant in cardiovascular pathology and pathophysiology. Superoxide anion, with its unpaired electron, binds rapidly to nitric oxide (NO), which also has an unpaired electron. Because NO is a critical vasodilator substance, the reaction between superoxide and NO effectively scavenges NO, reducing its bioavailability. This leads to vasoconstriction, increased platelet-endothelial cell adhesion, platelet aggregation and thrombus formation, increased leukocyte-endothelial cell adhesion, and morphological changes in blood vessels, such as cell proliferation.
When NO reacts with superoxide anion, peroxynitrite is created (ONOO-). Although this compound can produce cellular damage at high concentrations, it has also been shown to be a vasodilator through its ability to react with other compounds to produce new chemical entities that serve as NO donors.
Studies have shown that some antioxidants, such as vitamins C and E, and n-acetylcysteine, can reduce superoxide anion and lead to increased NO bioavailability.
Revised 11/03/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)