Cardiac Muscle Force-Velocity Relationship
Description of Force-Velocity Relationship
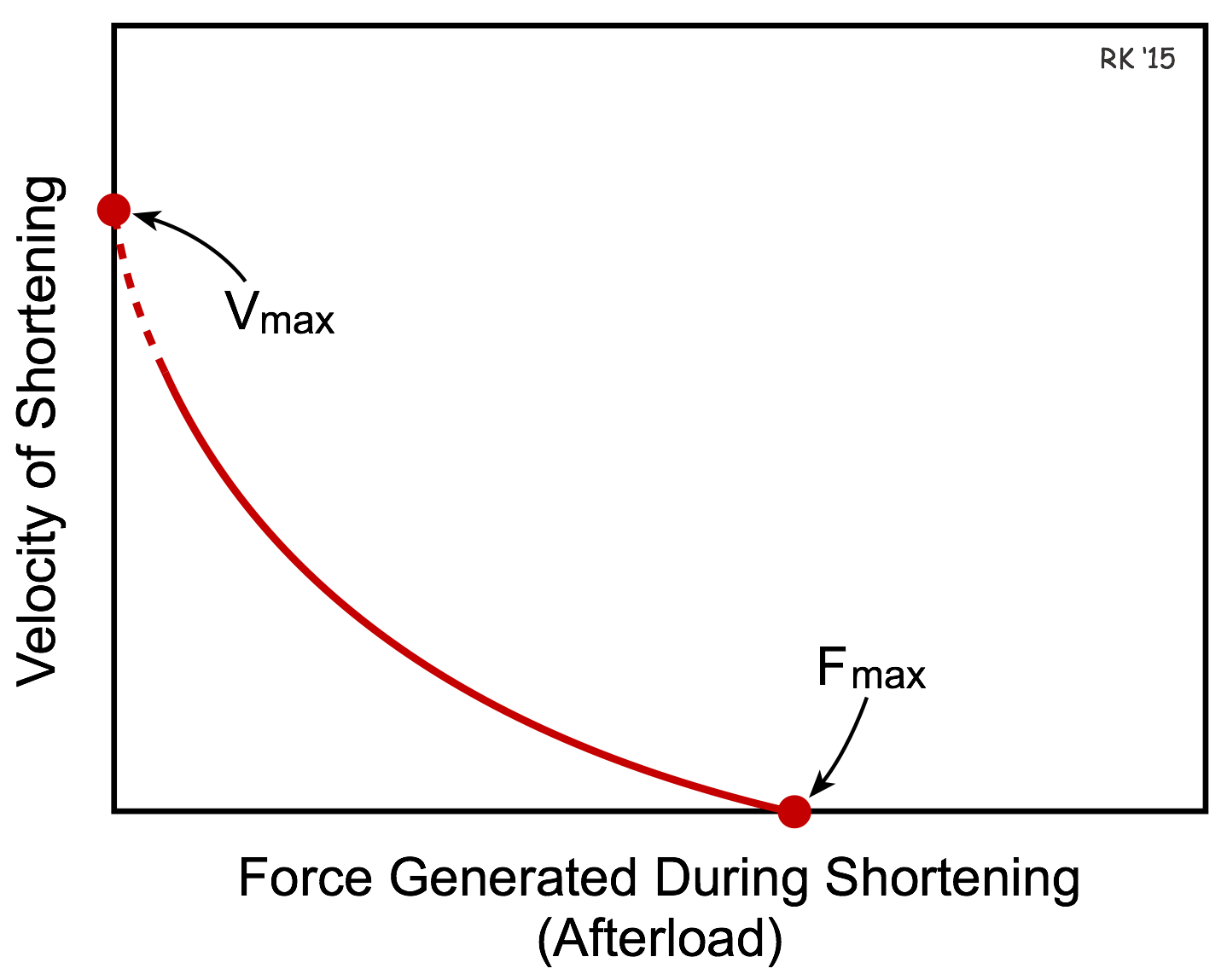 The length-tension relationship examines how changes in preload affect isometric tension development. When a muscle fiber contracts, it also shortens so that external work can be performed. If we were to isolate a piece of cardiac muscle and study the effects of afterload on the velocity of fiber shortening, we would find that the greater the afterload, the slower the velocity of shortening (see Figure). We all experience this, for example, when we lift heavy versus light objects. The heavier the object that we lift, the slower our muscles contract (i.e., shorten) despite a maximal voluntary contraction. Therefore, there is an inverse relationship between shortening velocity and afterload and this is termed the force-velocity relationship and is shown in the figure in which the velocity of muscle shortening is plotted against the force generated during shortening (muscle afterload).
The length-tension relationship examines how changes in preload affect isometric tension development. When a muscle fiber contracts, it also shortens so that external work can be performed. If we were to isolate a piece of cardiac muscle and study the effects of afterload on the velocity of fiber shortening, we would find that the greater the afterload, the slower the velocity of shortening (see Figure). We all experience this, for example, when we lift heavy versus light objects. The heavier the object that we lift, the slower our muscles contract (i.e., shorten) despite a maximal voluntary contraction. Therefore, there is an inverse relationship between shortening velocity and afterload and this is termed the force-velocity relationship and is shown in the figure in which the velocity of muscle shortening is plotted against the force generated during shortening (muscle afterload).
The x-intercept in the force-velocity relationship represents the point at which the afterload is so great that the muscle fiber cannot shorten, and therefore represents the maximal isometric force (Fmax). The y-intercept represents an extrapolated value for the maximal velocity of shortening (Vmax) that would be achieved if there were no afterload. The value for Vmax is extrapolated because it cannot be measured experimentally since the muscle cannot contract without a finite preload, which becomes the afterload during shortening in the absence of an additional afterload.
It is important to note that cardiac muscle fibers do not operate on a single force-velocity curve. This relationship is altered by changes in both preload and inotropy. The former shares some similarities with skeletal muscle; the latter, however, is unique to cardiac muscle.
How Preload Affects the Force-Velocity Relationship
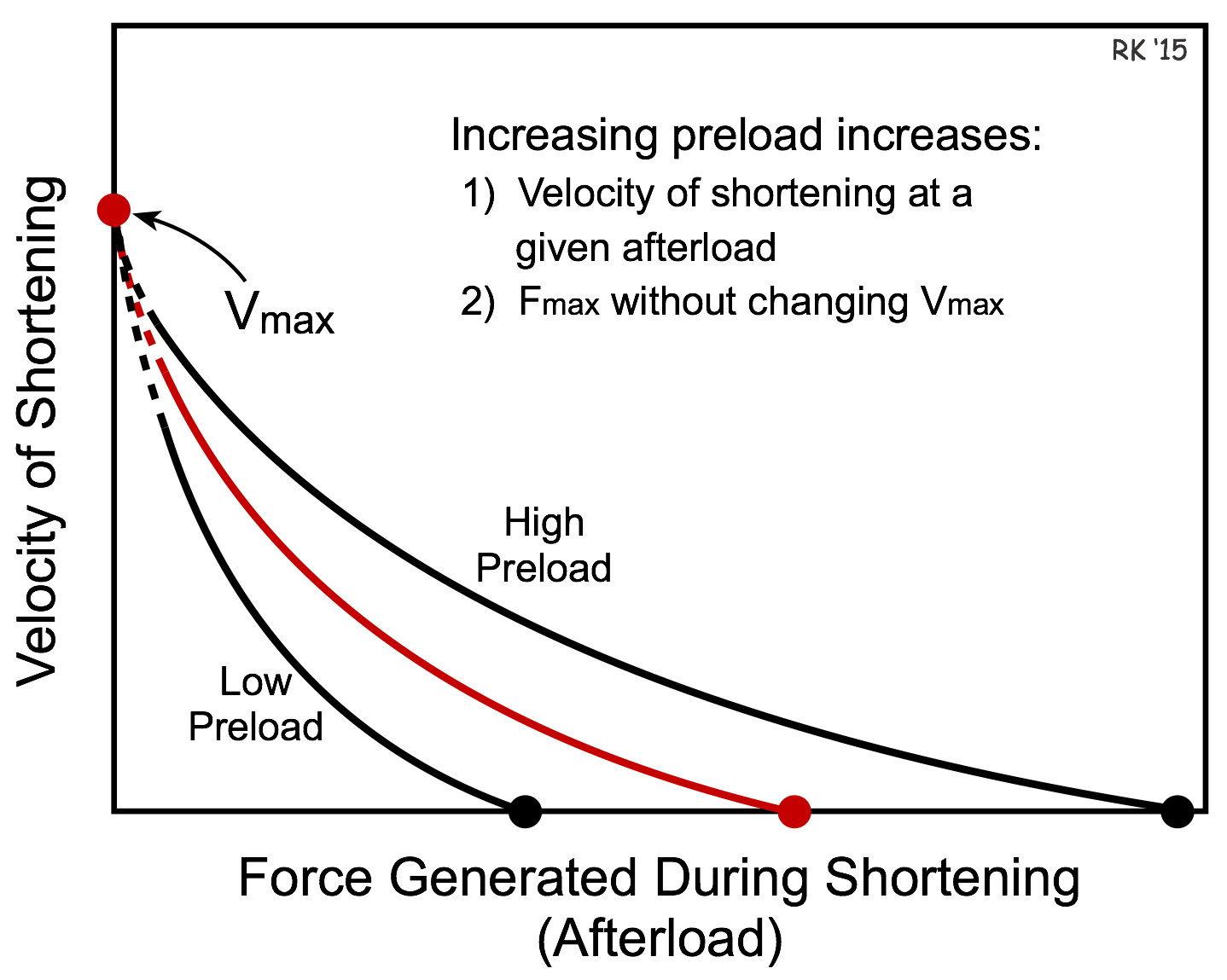 If preload is increased, cardiac muscle fibers will have a greater velocity of shortening at a given afterload (see figure). Conversely, if preload decreases, the velocity of shortening decreases at a given afterload. This occurs because the length-tension relationship dictates that as the preload is increased, there is an increase in active tension development. Once the fiber shortens, the increased tension generating capability at the increased preload results in a greater velocity of shortening. Therefore, increasing the preload enables muscle to contract faster (i.e., increased shortening velocity) against a given afterload. Note that increasing preload increases the maximal isometric force (Fmax) besides increasing the shortening velocity at a given afterload. Note, however, that changes in preload do not alter not alter Vmax.
If preload is increased, cardiac muscle fibers will have a greater velocity of shortening at a given afterload (see figure). Conversely, if preload decreases, the velocity of shortening decreases at a given afterload. This occurs because the length-tension relationship dictates that as the preload is increased, there is an increase in active tension development. Once the fiber shortens, the increased tension generating capability at the increased preload results in a greater velocity of shortening. Therefore, increasing the preload enables muscle to contract faster (i.e., increased shortening velocity) against a given afterload. Note that increasing preload increases the maximal isometric force (Fmax) besides increasing the shortening velocity at a given afterload. Note, however, that changes in preload do not alter not alter Vmax.
How Inotropy Affects the Force-Velocity Relationship
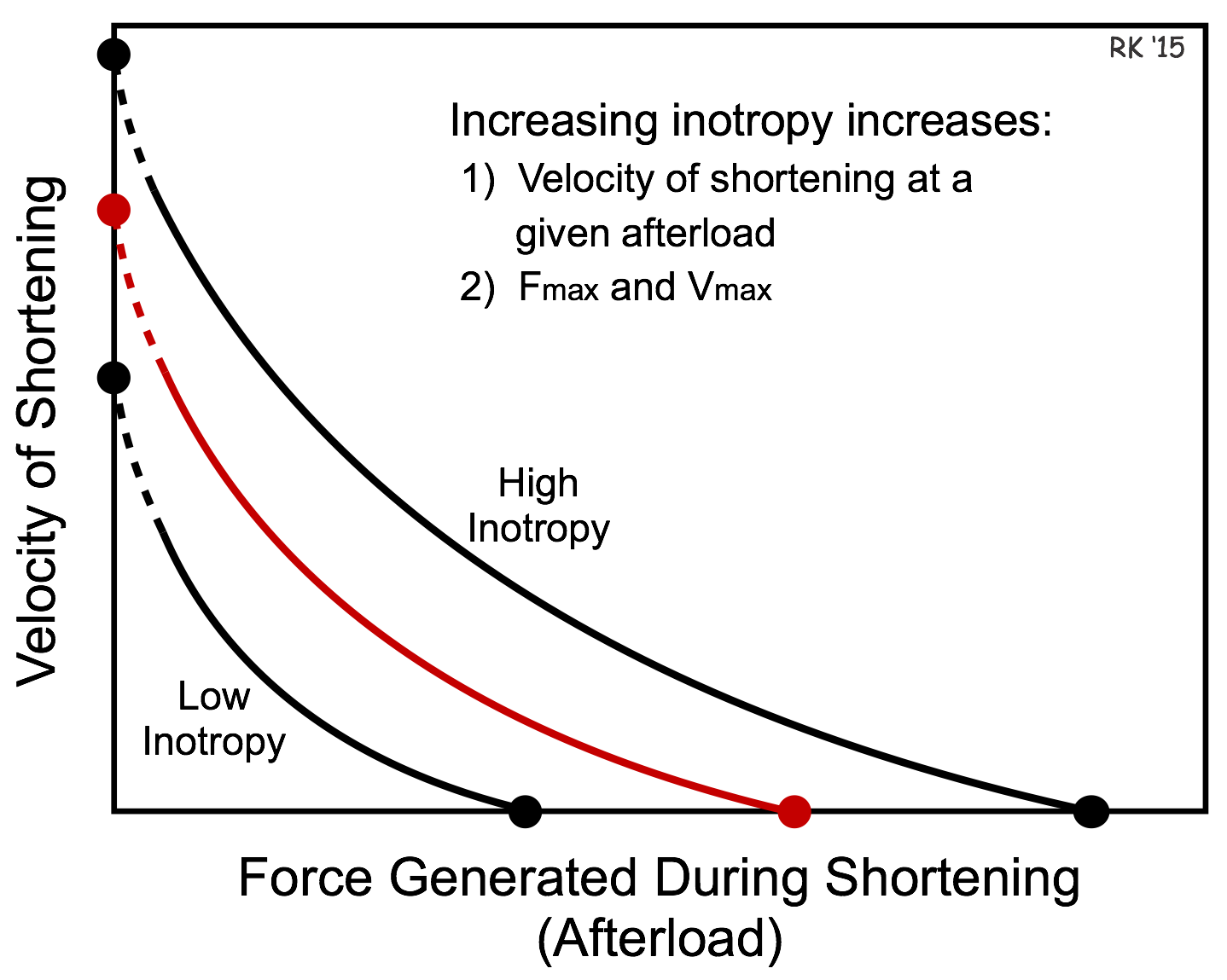 Changes in inotropy also alter the force-velocity relationship. If the inotropic state of the cardiac fiber is increased, there is a parallel shift in the force-velocity curve such that there is an increase in both Vmax and Fmax (see figure). The increase in velocity at any given preload results from the increased inotropy enhancing force generation by the actin and myosin filaments, and increasing the rate of cross bridge cycling. The increase in Vmax is noteworthy because Vmax represents the intrinsic capability of a muscle fiber to generate force independent of either preload or afterload. Therefore, Vmax is sometimes used experimentally as an index or measure of inotropy for cardiac muscle fibers.
Changes in inotropy also alter the force-velocity relationship. If the inotropic state of the cardiac fiber is increased, there is a parallel shift in the force-velocity curve such that there is an increase in both Vmax and Fmax (see figure). The increase in velocity at any given preload results from the increased inotropy enhancing force generation by the actin and myosin filaments, and increasing the rate of cross bridge cycling. The increase in Vmax is noteworthy because Vmax represents the intrinsic capability of a muscle fiber to generate force independent of either preload or afterload. Therefore, Vmax is sometimes used experimentally as an index or measure of inotropy for cardiac muscle fibers.
Revised 01/22/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)