Oxygen Transport and Diffusion
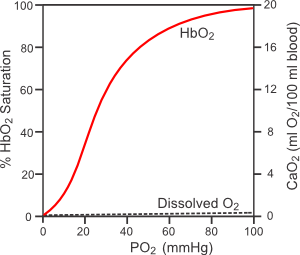 Most of the oxygen that is carried by blood is bound to hemoglobin found in red blood cells (erythrocytes). The binding of oxygen to hemoglobin (HbO2) is determined by the partial pressure of oxygen (PO2) and the affinity of the hemoglobin for oxygen, as shown in the figure to the right (hemoglobin-oxygen dissociation curve). At normal arterial PO2 values (~100 mmHg), the hemoglobin is about 97% saturated. The amount of oxygen that is bound to hemoglobin at normal arterial PO2 values is determined by the total amount of hemoglobin. At a normal red cell hematocrit of 45%, there is about 15 g of hemoglobin per 100 ml of blood. Each gram of hemoglobin can bind 1.34 ml of oxygen; therefore, there is normally about 19.5 ml O2/100 ml blood bound to hemoglobin in normal arterial blood (1.34 ml O2/g x 15 g x 0.97). There is also a small amount of oxygen that is dissolved in the free water of the plasma and cells (~0.3 ml O2/100 ml blood). When the bound and dissolved oxygen are added together, arterial blood normally contains about 20 ml O2/100 ml blood (20 vol%).
Most of the oxygen that is carried by blood is bound to hemoglobin found in red blood cells (erythrocytes). The binding of oxygen to hemoglobin (HbO2) is determined by the partial pressure of oxygen (PO2) and the affinity of the hemoglobin for oxygen, as shown in the figure to the right (hemoglobin-oxygen dissociation curve). At normal arterial PO2 values (~100 mmHg), the hemoglobin is about 97% saturated. The amount of oxygen that is bound to hemoglobin at normal arterial PO2 values is determined by the total amount of hemoglobin. At a normal red cell hematocrit of 45%, there is about 15 g of hemoglobin per 100 ml of blood. Each gram of hemoglobin can bind 1.34 ml of oxygen; therefore, there is normally about 19.5 ml O2/100 ml blood bound to hemoglobin in normal arterial blood (1.34 ml O2/g x 15 g x 0.97). There is also a small amount of oxygen that is dissolved in the free water of the plasma and cells (~0.3 ml O2/100 ml blood). When the bound and dissolved oxygen are added together, arterial blood normally contains about 20 ml O2/100 ml blood (20 vol%).
Because the hemoglobin-oxygen dissociation curve is sigmoidal, a reduction in arterial PO2 to 75 mmHg causes less than a 10% decrease in oxygen saturation and content in the blood. However, when blood is exposed to normal tissue PO2 levels of 20-40 mmHg, the hemoglobin cannot bind the oxygen as well, so the oxygen saturation falls considerably. Hemoglobin is only about 50% saturated with oxygen when the PO2 is 25 mmHg. The PO2 at which the hemoglobin is 50% saturated is the P50 value. This value can increase or decrease depending on several factors. For example, increased PCO2, decreased pH and increased temperature increase the P50 by shifting the hemoglobin-oxygen dissociation curve to the right. This means that at any PO2, the amount of oxygen bound to hemoglobin is reduced. This shift in the curve contributes to the unloading of oxygen from hemoglobin under conditions of increased oxygen demand by the tissue. Increased tissue oxygen consumption is accompanied by increased PCO2, decreased pH and increased temperature in the tissue surrounding the blood vessels.
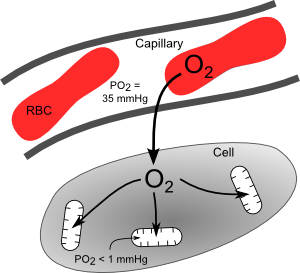 Oxygen rapidly diffuses from the blood into the tissues at the level of the microcirculation, particularly at capillaries (see figure). Because oxygen is highly lipid soluble, it readily passes through cell membranes. The rate of oxygen diffusion is determined primarily by the PO2 difference between the plasma and cells surrounding the capillaries, as described by Fick's first law of diffusion. Although there is considerable heterogeneity in PO2 values among different capillaries, a typical value ranges from 30 to 40 mmHg. The PO2 inside cells is very low because oxygen is consumed by mitochondria within the cells. The PO2 inside mitochondria is less than 1 mmHg because these are the organelles that consume the oxygen to generate ATP. With increased oxidative metabolism of a tissue, the mitochondria need to make more ATP and therefore consume more oxygen. This oxygen diffuses from the plasma, across the capillary endothelium and interstitial space, then into the cell and its mitochondria.
Oxygen rapidly diffuses from the blood into the tissues at the level of the microcirculation, particularly at capillaries (see figure). Because oxygen is highly lipid soluble, it readily passes through cell membranes. The rate of oxygen diffusion is determined primarily by the PO2 difference between the plasma and cells surrounding the capillaries, as described by Fick's first law of diffusion. Although there is considerable heterogeneity in PO2 values among different capillaries, a typical value ranges from 30 to 40 mmHg. The PO2 inside cells is very low because oxygen is consumed by mitochondria within the cells. The PO2 inside mitochondria is less than 1 mmHg because these are the organelles that consume the oxygen to generate ATP. With increased oxidative metabolism of a tissue, the mitochondria need to make more ATP and therefore consume more oxygen. This oxygen diffuses from the plasma, across the capillary endothelium and interstitial space, then into the cell and its mitochondria.
Although it is the PO2 difference and not the blood content of oxygen that drives diffusion, the hemoglobin-bound oxygen acts as a reservoir for oxygen. As oxygen diffuses from the plasma, the plasma and red cell cytoplasmic PO2 falls, which leads to oxygen dissociating from the hemoglobin and entering the dissolved pool of oxygen in the plasma from where it diffuses into the surrounding tissue. More oxygen bound to the hemoglobin means that more oxygen is available for diffusion into cells. Therefore, the amount of oxygen bound to hemoglobin is a major factor in determining oxygen delivery to a tissue.
Revised 12/29/2022
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)