Hydrostatic and Oncotic Pressures
There are two hydrostatic and two oncotic pressures that affect transcapillary fluid exchange. Click on the following links to learn more about these pressures:
Capillary Hydrostatic Pressure (PC)
This pressure drives fluid out of the capillary (i.e., filtration), and is highest at the arteriolar end of the capillary and lowest at the venular end. Depending upon the organ, the pressure may drop along the length of the capillary by 15-30 mmHg (axial or longitudinal pressure gradient). The axial gradient favors filtration at the arteriolar end (where PC is greatest) and reabsorption at the venular end of the capillary (where PC is the lowest). The average capillary hydrostatic pressure is determined by arterial and venous pressures (PA and PV), and by the ratio of post-to-precapillary resistances (RV/RA). An increase in either arterial or venous pressure will increase capillary pressure; however, a change in PA is only about one-fifth as effective in changing PC as the same absolute change in PV. Because venous resistance is relatively low, changes in PV are readily transmitted back to the capillary, and conversely because arterial resistance is relatively high, changes in PA are poorly transmitted downstream to the capillary. Therefore, PC is much more influenced by changes in PV than by changes in PA. Furthermore, PC is increased by precapillary vasodilation (particularly by arteriolar dilation), whereas precapillary vasoconstriction decreases PC. Venous constriction increases PC, whereas venous dilation decreases PC.
The effects of arterial and venous pressures and resistances on PC are summarized in the following relationship:
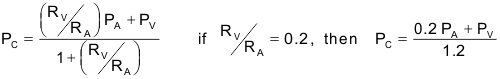
The above expression is derived from a simple model comprising a series-coupled pre- and postcapillary resistance. In many tissues, the post-to-precapillary resistance ratio is about 0.2, so precapillary resistance (mostly arteriolar) is about 5-times greater than postcapillary (venular) resistance. When this ratio is 0.2, a change in arterial pressure is only about one-fifth as effective in changing capillary pressure as a comparable change in venous pressure. If this ratio increases, as occurs with arteriolar vasodilation, then arterial pressure has a greater influence on capillary pressure, which rises. Conversely, arteriolar constriction decreases this ratio and decreases capillary pressure.
Tissue (Interstitial) Pressure (Pi)
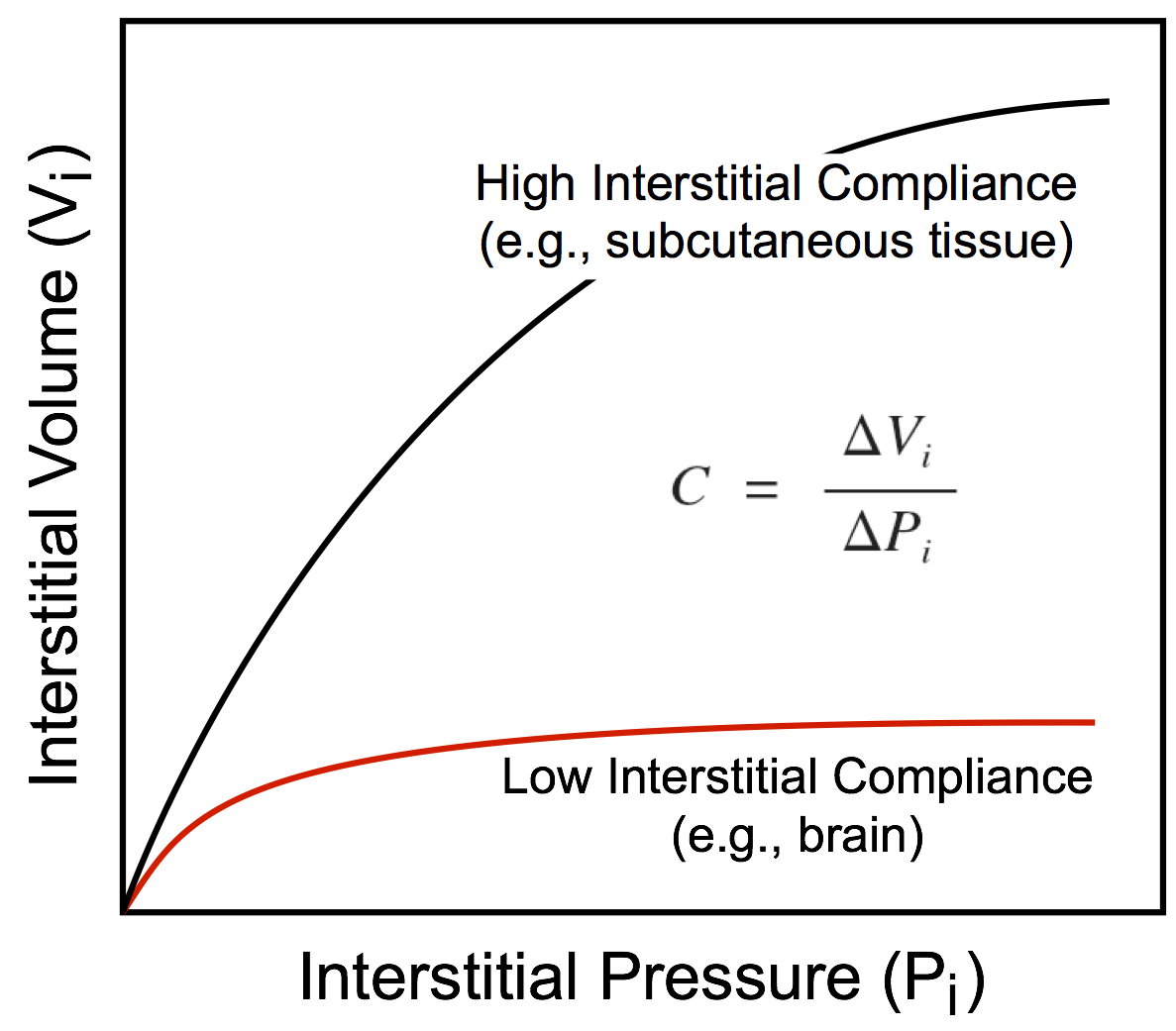 This hydrostatic pressure is determined by the interstitial fluid volume and the compliance of the tissue interstitium, which is defined as the change in volume divided by the change in pressure. The more fluid that filters into the interstitium, the greater the volume of the interstitial space (Vi) and the hydrostatic pressure within that space (Pi). In some organs, the interstitial compliance is low, so small increases in interstitial volume lead to large increases in pressure. Examples of this include the brain and kidney, which are encased by rigid bone (brain) or by a capsule (kidney). In contrast, soft tissues such as skin, muscle, and lung have a high compliance and therefore the interstitial space can undergo a large expansion with a relatively small increase in pressure. As interstitial volume increases, interstitial pressure increases, which can limit the amount of filtration into the interstitium because this pressure opposes the capillary hydrostatic pressure. Therefore, as the hydrostatic pressure gradient (Pc – Pi) decreases because of the increase in interstitial pressure, fluid filtration will be reduced. However, large increases in tissue interstitial pressure can lead to tissue damage and cellular death. Normally, Pi is near zero. In some tissues, it is slightly subatmospheric, whereas in others it is slightly positive.
This hydrostatic pressure is determined by the interstitial fluid volume and the compliance of the tissue interstitium, which is defined as the change in volume divided by the change in pressure. The more fluid that filters into the interstitium, the greater the volume of the interstitial space (Vi) and the hydrostatic pressure within that space (Pi). In some organs, the interstitial compliance is low, so small increases in interstitial volume lead to large increases in pressure. Examples of this include the brain and kidney, which are encased by rigid bone (brain) or by a capsule (kidney). In contrast, soft tissues such as skin, muscle, and lung have a high compliance and therefore the interstitial space can undergo a large expansion with a relatively small increase in pressure. As interstitial volume increases, interstitial pressure increases, which can limit the amount of filtration into the interstitium because this pressure opposes the capillary hydrostatic pressure. Therefore, as the hydrostatic pressure gradient (Pc – Pi) decreases because of the increase in interstitial pressure, fluid filtration will be reduced. However, large increases in tissue interstitial pressure can lead to tissue damage and cellular death. Normally, Pi is near zero. In some tissues, it is slightly subatmospheric, whereas in others it is slightly positive.
Capillary Plasma Oncotic Pressure (ΠC)
Because the capillary barrier is readily permeable to ions, the osmotic pressure within the capillary is principally determined by plasma proteins that are relatively impermeable. Therefore, instead of speaking of "osmotic" pressure, this pressure is referred to as the "oncotic" pressure or "colloid osmotic" pressure because it is generated by colloids. Albumin generates about 70% of the oncotic pressure. This pressure is typically 25-30 mmHg. The oncotic pressure increases along the length of the capillary, particularly in capillaries having high net filtration (e.g., in renal glomerular capillaries) because the filtering fluid leaves behind proteins, leading to an increase in protein concentration.
Normally, when oncotic pressure is measured, it is measured across a semipermeable membrane that is permeable to fluid and electrolytes but not to large protein molecules. In most capillaries, however, the wall (primarily endothelium) has a finite permeability to proteins. Membrane permeability to proteins depends on the type of capillary and the nature of the protein (size, shape, charge). Because of this finite permeability, the effective oncotic pressure generated across the capillary membrane is less than that calculated from the protein concentration. The effects of finite protein permeability on the physiological oncotic pressure can be determined by knowing the reflection coefficient (σ) of the capillary wall. If the capillary is impermeable to protein, then σ = 1. If the capillary is freely permeable to protein, then σ = 0. Continuous capillaries have a high σ (>0.9), whereas discontinuous and fenestrated capillaries that are very "leaky" to proteins have a relatively low σ. When the value for σ is very low, plasma and tissue oncotic pressures may have a negligible influence on the net driving force.
Tissue (interstitial) Oncotic Pressure (Πi)
The effective oncotic pressure of the interstitial fluid depends on the interstitial protein concentration and the reflection coefficient of the capillary wall. The more permeable the capillary barrier is to plasma proteins, the higher the interstitial protein concentration; however, the effective oncotic pressure will be less than that calculated from the protein concentration because of the lower reflection coefficient. Interstitial protein concentration can also be determined by the amount of fluid filtration into the interstitium. For example, increased capillary filtration dilutes the interstitial protein concentration and therefore reduces the oncotic pressure exerted by those proteins. A reduction in the interstitial oncotic pressure increases the net oncotic pressure across the capillary endothelium (πC - πi), which opposes filtration and promotes reabsorption. This serves as a mechanism to limit capillary filtration. In a "typical" tissue, tissue oncotic pressure is about 5 mmHg, which is much lower than the capillary plasma oncotic pressure.
Revised 11/05/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)